K
Kathleen Martin
Guest
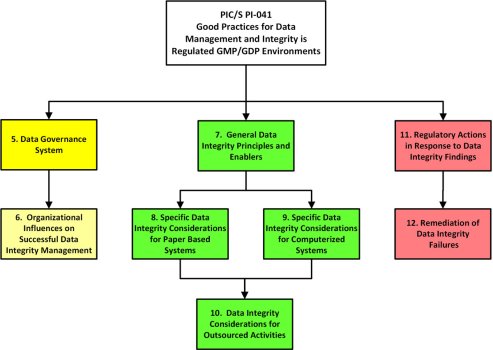
Owing to widespread data falsification and poor data management practices, data integrity and compliance with good manufacturing practice (GMP) regulations are currently a major topic in the pharmaceutical industry. To aid our understanding of data integrity concerns, regulatory authorities such as the World Health Organization (WHO),1 Medicines and Healthcare products Regulatory Agency (MHRA)2,3 and the US Food and Drug Administration (FDA)4 have issued guidance documents on the topic. In July 2021, the latest guidance document was released by the Pharmaceutical Inspection Cooperation Scheme (PIC/S) entitled Good Practices for Data Management and Integrity in Regulated GMP/GDP Environments.1 This article will give an overview of the whole guidance document and review specific requirements for computerized systems. This is an important document as it is written by inspectors for inspectors.

What is PIC/S?
A word of explanation is needed about PIC/S. This organization is essentially a good manufacturing practice (GMP) inspector’s club of over 50 regulatory authorities. PIC/S’s aim is to harmonize GMP regulations globally. A regulatory authority applies to join PIC/S and after an assessment by other members is admitted if they meet the organization’s criteria. PIC/S has its own GMP regulations which are adopted by their members e.g., EU member states, UK, Australia and Japan; the exception being the FDA that still uses 21 CFR 211. In addition, PIC/S publishes regulatory guidance documents. One of these is PI-041 on data integrity. This guidance has had a long gestation, with the first draft issued in 2016, the next in 2018 for public comment and the final version released in July 2021.1
European Compliance Academy review of the 2018 draft guidance
Following the issue of the third draft – PI-041 – for public comment, the European Compliance Academy (ECA) held a meeting in February 2019 in Berlin with 25 of its members to review and submit comments to the PIC/S secretariat. Two of the ECA members were Wolfgang Schumacher, formerly of Roche and Yves Samson from Kereon AG, a consulting company.
Continue reading: https://www.technologynetworks.com/informatics/articles/the-latest-regulatory-guidance-for-data-integrity-and-regulatory-compliance-353714

What is PIC/S?
A word of explanation is needed about PIC/S. This organization is essentially a good manufacturing practice (GMP) inspector’s club of over 50 regulatory authorities. PIC/S’s aim is to harmonize GMP regulations globally. A regulatory authority applies to join PIC/S and after an assessment by other members is admitted if they meet the organization’s criteria. PIC/S has its own GMP regulations which are adopted by their members e.g., EU member states, UK, Australia and Japan; the exception being the FDA that still uses 21 CFR 211. In addition, PIC/S publishes regulatory guidance documents. One of these is PI-041 on data integrity. This guidance has had a long gestation, with the first draft issued in 2016, the next in 2018 for public comment and the final version released in July 2021.1
European Compliance Academy review of the 2018 draft guidance
Following the issue of the third draft – PI-041 – for public comment, the European Compliance Academy (ECA) held a meeting in February 2019 in Berlin with 25 of its members to review and submit comments to the PIC/S secretariat. Two of the ECA members were Wolfgang Schumacher, formerly of Roche and Yves Samson from Kereon AG, a consulting company.
Continue reading: https://www.technologynetworks.com/informatics/articles/the-latest-regulatory-guidance-for-data-integrity-and-regulatory-compliance-353714